Science Chemistry Chemistry questions and answers Rank the following from most to least acidic. Rank from most to least acidic. To rank items as equivalent, overlap them. pH=3 pH=3.5 [H3O+]= 10^-2 pH=10 [H3O+]=10^-4 This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts.
Rank the following acids in decreasing order of their acid streng… | Channels for Pearson+
What is the molar concentration of [H3O+] in a cola that has a pH of 3.120? (For help with significant figures,, Part C – The relationship between pH and acidityPart complete Rank the following from most acidic to least acidic. Rank these items from most acidic to least acidic. To rank items as equivalent, overlap them. and more.

Source Image: pearson.com
Download Image
Step 1 pH = 3 Explanation: pH = 3 (most acidic) – Lowest pH value indicates high acidity. [H3O+] = 10^-2 View the full answer Answer Unlock Previous question Next question Transcribed image text: Ranik the following from most acidic to least acidic. Rank these items from most acidic to least acidic. To rank items as equivalent, overlap them.
Source Image: chegg.com
Download Image
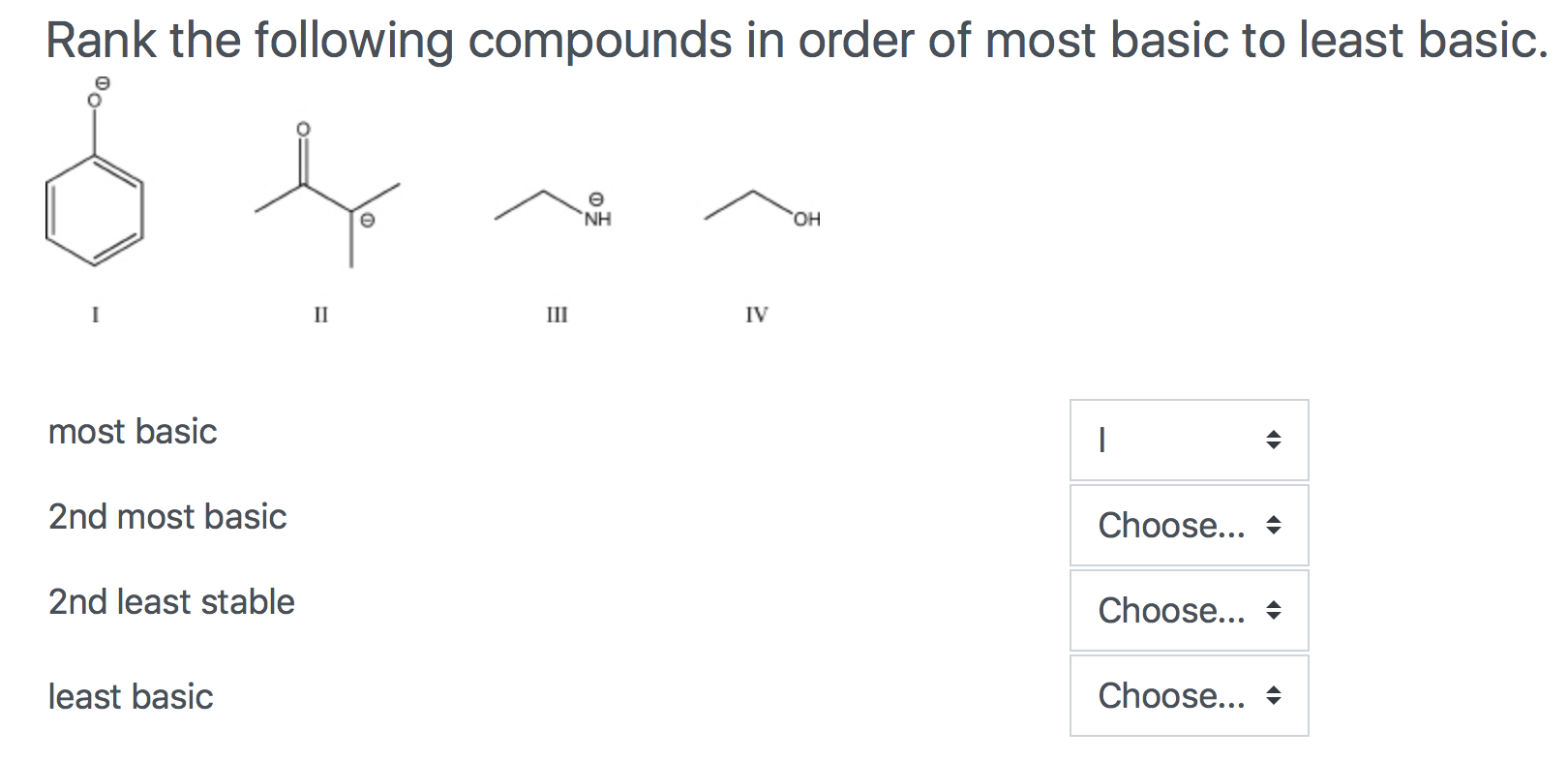
Rank the compounds in each set in order of increasing acid streng… | Channels for Pearson+ Rank the ions (−CH3, −NH2, HO−, and F−) from most basic to least basic. a. Rank the following alcohols from strongest to weakest acid. CH2═CHCH2OH CH3CH2CH2OH HC≡CCH2OH … Rank the indicated hydrogen in the following compounds from most acidic to least acidic: Which species in each of the pairs in Problem 80 is the stronger base? a.
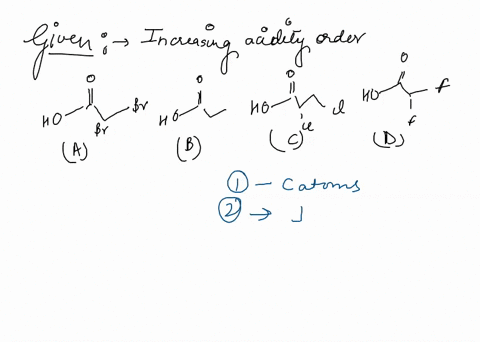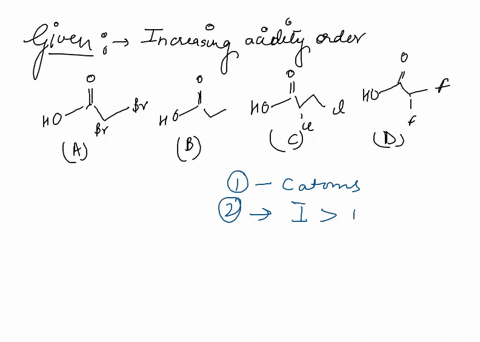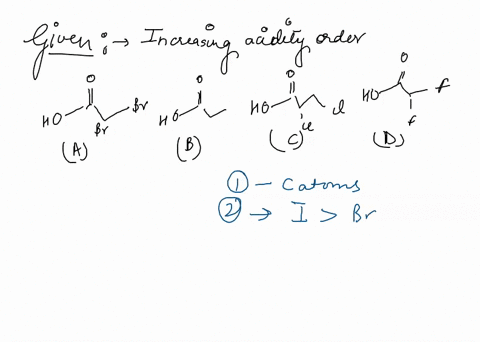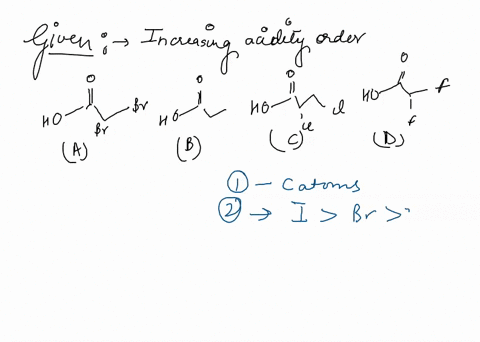
Source Image: numerade.com
Download Image
Rank The Following From Most To Least Acidic
Rank the ions (−CH3, −NH2, HO−, and F−) from most basic to least basic. a. Rank the following alcohols from strongest to weakest acid. CH2═CHCH2OH CH3CH2CH2OH HC≡CCH2OH … Rank the indicated hydrogen in the following compounds from most acidic to least acidic: Which species in each of the pairs in Problem 80 is the stronger base? a. 40. Question 2n. Textbook Question. The following compounds are listed in increasing order of acidity. In each case, the most acidic proton is shown in red. W pKa=25 X pKa= 23Y pKa= 8.8 Z pKa= 4.2 b. Explain why X is a stronger acid than W. c. Explain why Y is a stronger acid than X. d. Explain why Z is a stronger acid than Y.
SOLVED: Rank the following compounds according to increasing acidity: (least acidic to most acidic) ClOH COH OH AS B (least acidic) (most acidic) Hyc-H FaC-H HacC B (least acidic) (most acidic)
7 PRACTICE PROBLEM Identify the anion that would more readily react with H+. 8 PRACTICE PROBLEM There are four compounds given in increasing order of acidity. The most acidic hydrogen in each of these structures is circled. Explain why each of these compounds is more acidic than the preceding one. 9 PRACTICE PROBLEM Solved 6) Rank the following from most to least acidic | Chegg.com
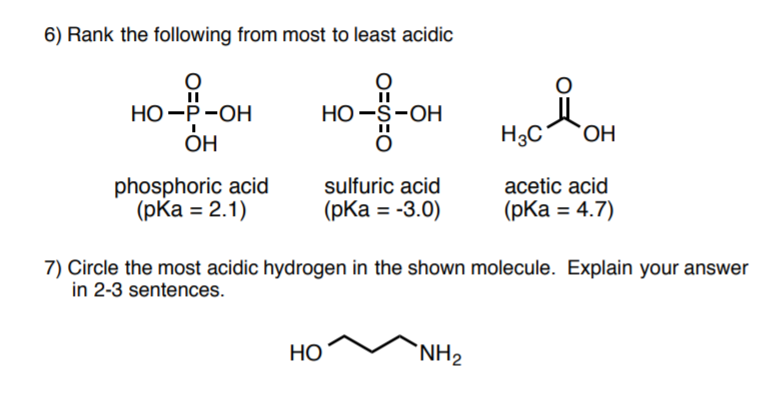
Source Image: chegg.com
Download Image
Rank the following phenols from most to least acidic. Briefly justify your answer. | Homework.Study.com 7 PRACTICE PROBLEM Identify the anion that would more readily react with H+. 8 PRACTICE PROBLEM There are four compounds given in increasing order of acidity. The most acidic hydrogen in each of these structures is circled. Explain why each of these compounds is more acidic than the preceding one. 9 PRACTICE PROBLEM

Source Image: homework.study.com
Download Image
Rank the following acids in decreasing order of their acid streng… | Channels for Pearson+ Science Chemistry Chemistry questions and answers Rank the following from most to least acidic. Rank from most to least acidic. To rank items as equivalent, overlap them. pH=3 pH=3.5 [H3O+]= 10^-2 pH=10 [H3O+]=10^-4 This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts.

Source Image: pearson.com
Download Image
Rank the compounds in each set in order of increasing acid streng… | Channels for Pearson+ Step 1 pH = 3 Explanation: pH = 3 (most acidic) – Lowest pH value indicates high acidity. [H3O+] = 10^-2 View the full answer Answer Unlock Previous question Next question Transcribed image text: Ranik the following from most acidic to least acidic. Rank these items from most acidic to least acidic. To rank items as equivalent, overlap them.

Source Image: pearson.com
Download Image
SOLVED: 11. Rank the following carboxylic acids in order of acidity from least to most acidic OH OH OH OH Cl OH NOz OH OH OH OH Jul 9, 2022Rank the following solutions in order of increasing acidity, placing the most acidic solution at the left. (CH3COOH is approximately 1.0% ionized at this concentration.) pH=7.45 pH=0.00 [HCL]=0.15 M [CH_3COOH]=0.15 M Follow • 2 Add comment Report 1 Expert Answer Best Newest Oldest J.R. S. answered • 07/09/22 Tutor 5.0 (145)
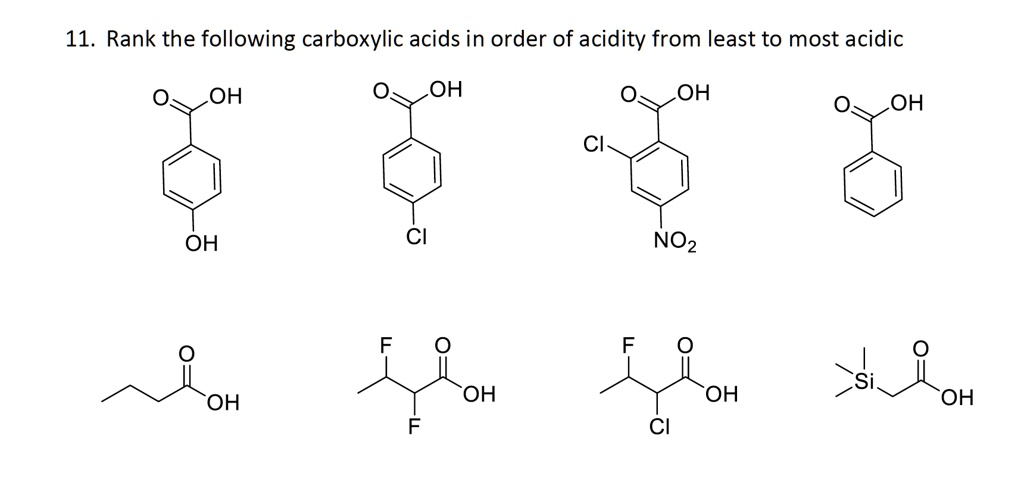
Source Image: numerade.com
Download Image
Ranking Acidity Practice Problems | Channels for Pearson+ Rank the ions (−CH3, −NH2, HO−, and F−) from most basic to least basic. a. Rank the following alcohols from strongest to weakest acid. CH2═CHCH2OH CH3CH2CH2OH HC≡CCH2OH … Rank the indicated hydrogen in the following compounds from most acidic to least acidic: Which species in each of the pairs in Problem 80 is the stronger base? a.

Source Image: pearson.com
Download Image
Rank the following molecules from most to least acidic? – Organic Chemistry – Science Forums 40. Question 2n. Textbook Question. The following compounds are listed in increasing order of acidity. In each case, the most acidic proton is shown in red. W pKa=25 X pKa= 23Y pKa= 8.8 Z pKa= 4.2 b. Explain why X is a stronger acid than W. c. Explain why Y is a stronger acid than X. d. Explain why Z is a stronger acid than Y.
.jpg.912553b555b97bbe28e5a08cb99f66e9.jpg)
Source Image: scienceforums.net
Download Image
Rank the following phenols from most to least acidic. Briefly justify your answer. | Homework.Study.com
Rank the following molecules from most to least acidic? – Organic Chemistry – Science Forums What is the molar concentration of [H3O+] in a cola that has a pH of 3.120? (For help with significant figures,, Part C – The relationship between pH and acidityPart complete Rank the following from most acidic to least acidic. Rank these items from most acidic to least acidic. To rank items as equivalent, overlap them. and more.
Rank the compounds in each set in order of increasing acid streng… | Channels for Pearson+ Ranking Acidity Practice Problems | Channels for Pearson+ Jul 9, 2022Rank the following solutions in order of increasing acidity, placing the most acidic solution at the left. (CH3COOH is approximately 1.0% ionized at this concentration.) pH=7.45 pH=0.00 [HCL]=0.15 M [CH_3COOH]=0.15 M Follow • 2 Add comment Report 1 Expert Answer Best Newest Oldest J.R. S. answered • 07/09/22 Tutor 5.0 (145)