Mar 25, 2022Tutor 5.0 (145) Ph.D. University Professor with 10+ years Tutoring Experience About this tutor › Write the reaction: KOH + HBrO ==> KBrO + H 2 O moles KOH present = 40.0 ml x 1 L / 1000 ml x 0.150 mol/L = 0.006 moles KOH moles HBrO present = 20.0 ml x 1 L / 1000 ml x 0.300 mol/L = 0.006 moles HBrO
SOLVED: Calculate the pH of a 0.160 M solution of KOH. Part 2 (1 point) Calculate the pOH of a 0.160 M solution of KOH.
Biology Stomach Question What is the pH of a 0.05 M solution of KOH ? A 12.7 B 1.3 C 5.2 D 9 Solution Verified by Toppr Was this answer helpful? 0 Similar Questions Q 1 What will be the P H value of 0.05 M 2 Ba(OH)2 solution? View Solution Q 2 As the temperature increases, the pH of a KOH solution: View Solution Q 3
![Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download](https://images.slideplayer.com/39/10846268/slides/slide_3.jpg)
Source Image: slideplayer.com
Download Image
Calculate the pH of a solution prepared by adding 45.0 mL of a 0.213 M HCl solution to 125.0 mL of a 0.150 M solution of ammonia. The \(pK_b\) of ammonia is 4.75 at 25°C. Answer. 9.23 (Note that since the ammonia is approximately half-neutralized at this point, this pH is very close to the \(pK_a\) of ammonium, 9.25!)

Source Image: youtube.com
Download Image
If 0-561 g of KOH is dissolved in water to give 200 mL of solution at 298 K. Calculate the concentrations of potassium, hydrogen and hydroxyl ions. What is its pH? – To find the concentration of a solution, we need to use the molarity (M) equation. molarity = mass/volume So, to find the concentration of 0.150 M KOH, we would use the following equation: concentration = (0.150 M)*(1 mole/L) Now that we have the concentration, we can use the pH equation to find the pH.

Source Image: m.youtube.com
Download Image
Calculate The Ph Of A 0.150 M Solution Of Koh
To find the concentration of a solution, we need to use the molarity (M) equation. molarity = mass/volume So, to find the concentration of 0.150 M KOH, we would use the following equation: concentration = (0.150 M)*(1 mole/L) Now that we have the concentration, we can use the pH equation to find the pH. Track your food intake, exercise, sleep and meditation for free. Enter components of a solution to calculate pH. pKw: Compute pH. Instructions for pH Calculator. Case 1. Initial concentrations of components in a mixture are known. For each compound enter compound name (optional), concentration and Ka/Kb or pKa/pKb values.
Calculate pH of a KOH Solution 001 – YouTube
There are two calculators – one for either strong acid or strong base, and another for either weak acid or weak base. Below you can find two calculators that you can use to check answers to chemistry problems. If 25 mL of 0.45 M HCl is added to 30 mL of 0.35 M NaOH, what is the pH of the final solution? – Quora
Source Image: quora.com
Download Image
Tty | PDF | Buffer Solution | Acid Dissociation Constant There are two calculators – one for either strong acid or strong base, and another for either weak acid or weak base. Below you can find two calculators that you can use to check answers to chemistry problems.

Source Image: scribd.com
Download Image
SOLVED: Calculate the pH of a 0.160 M solution of KOH. Part 2 (1 point) Calculate the pOH of a 0.160 M solution of KOH. Mar 25, 2022Tutor 5.0 (145) Ph.D. University Professor with 10+ years Tutoring Experience About this tutor › Write the reaction: KOH + HBrO ==> KBrO + H 2 O moles KOH present = 40.0 ml x 1 L / 1000 ml x 0.150 mol/L = 0.006 moles KOH moles HBrO present = 20.0 ml x 1 L / 1000 ml x 0.300 mol/L = 0.006 moles HBrO
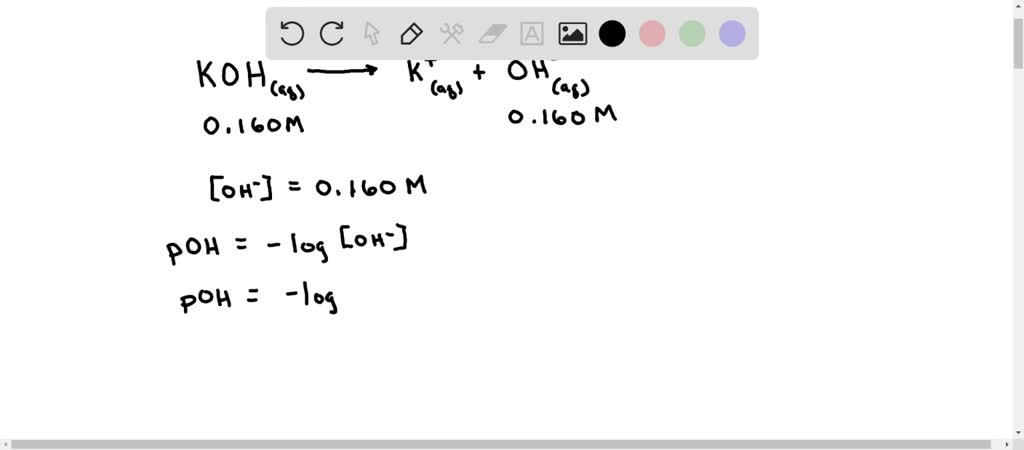
Source Image: numerade.com
Download Image
If 0-561 g of KOH is dissolved in water to give 200 mL of solution at 298 K. Calculate the concentrations of potassium, hydrogen and hydroxyl ions. What is its pH? – Calculate the pH of a solution prepared by adding 45.0 mL of a 0.213 M HCl solution to 125.0 mL of a 0.150 M solution of ammonia. The \(pK_b\) of ammonia is 4.75 at 25°C. Answer. 9.23 (Note that since the ammonia is approximately half-neutralized at this point, this pH is very close to the \(pK_a\) of ammonium, 9.25!)
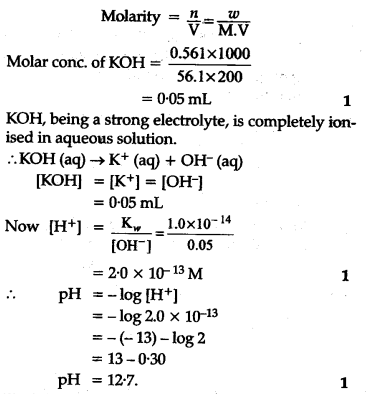
Source Image: ask.learncbse.in
Download Image
Calculate the pH of Buffer with added KOH – YouTube When the pH of the solution is actually between 6.8 and 7.2. In these cases, your assumption that water auto-ionizes to create exactly $\ce[HO^-] = 1.0\times10^-7 M $ will have significant figure implications).

Source Image: youtube.com
Download Image
Chem Problem Solving .5 2235 | PDF | Mole (Unit) | Iron To find the concentration of a solution, we need to use the molarity (M) equation. molarity = mass/volume So, to find the concentration of 0.150 M KOH, we would use the following equation: concentration = (0.150 M)*(1 mole/L) Now that we have the concentration, we can use the pH equation to find the pH.

Source Image: scribd.com
Download Image
Aqueous Acid–Base Equilibriums Track your food intake, exercise, sleep and meditation for free. Enter components of a solution to calculate pH. pKw: Compute pH. Instructions for pH Calculator. Case 1. Initial concentrations of components in a mixture are known. For each compound enter compound name (optional), concentration and Ka/Kb or pKa/pKb values.

Source Image: 2012books.lardbucket.org
Download Image
Tty | PDF | Buffer Solution | Acid Dissociation Constant
Aqueous Acid–Base Equilibriums Biology Stomach Question What is the pH of a 0.05 M solution of KOH ? A 12.7 B 1.3 C 5.2 D 9 Solution Verified by Toppr Was this answer helpful? 0 Similar Questions Q 1 What will be the P H value of 0.05 M 2 Ba(OH)2 solution? View Solution Q 2 As the temperature increases, the pH of a KOH solution: View Solution Q 3
If 0-561 g of KOH is dissolved in water to give 200 mL of solution at 298 K. Calculate the concentrations of potassium, hydrogen and hydroxyl ions. What is its pH? – Chem Problem Solving .5 2235 | PDF | Mole (Unit) | Iron When the pH of the solution is actually between 6.8 and 7.2. In these cases, your assumption that water auto-ionizes to create exactly $\ce[HO^-] = 1.0\times10^-7 M $ will have significant figure implications).