May 26, 2023= 207.2 + 70.9 = 278.1 g/mol Hence the Molar mass of PbCl2 is 278.1 g/mol. I hope you have understood the short and simple calculation for finding the molar mass of PbCl2. Remember In some books, you may see the unit of molar mass as grams/mole or g/mole. But all these units (i.e g/mol, grams/mole and g/mole) are the same.
⏩SOLVED:How many formula units are present in 500.0 g of lead(II)… | Numerade
Mg2+Cl−Cl− (4.3.2) (4.3.2) Mg 2 + Cl − Cl −. Now the positive and negative charges are balanced. We could write the chemical formula for this ionic compound as MgClCl MgClCl, but the convention is to use a numerical subscript when there is more than one ion of a given type— MgCl2 MgCl 2.

Source Image: scribd.com
Download Image
In this video we’ll write the correct formula for Lead (II) chloride, PbCl2.To write the formula for Lead (II) chloride we’ll use the Periodic Table and foll

Source Image: youtube.com
Download Image
SOLVED: The Ksp for lead chloride PbCl2 is 1.6 x 10^-5. Calculate the solubility of lead chloride in each of the following: a) water Solubility: 0.0158 mol/L b) 0.27 M Pb(NO3)2 Solubility: Structure, properties, spectra, suppliers and links for: Lead(II) chloride, 7758-95-4, PbCl2. Jump to main content Jump to site nav. Home; About us; Membership & professional community; Campaigning & outreach; Journals, books & databases … Molecular Formula Cl 2 Pb; Average mass 278.106 Da; Monoisotopic mass 277.914307 Da; ChemSpider ID 22867

Source Image: numerade.com
Download Image
How Many Formula Units Is 213 G Of Pbcl2
Structure, properties, spectra, suppliers and links for: Lead(II) chloride, 7758-95-4, PbCl2. Jump to main content Jump to site nav. Home; About us; Membership & professional community; Campaigning & outreach; Journals, books & databases … Molecular Formula Cl 2 Pb; Average mass 278.106 Da; Monoisotopic mass 277.914307 Da; ChemSpider ID 22867 Calculations: Formula: PbCl2 Molar Mass: 278.106 g/mol 1g=3.59575126031082E-03 mol Percent composition (by mass): Element Count Atom Mass % (by mass) Pb 1 207.2 74.5% Cl 2 35.453 25.5% Top Use: Molar Mass Calculator Formula: Co2 (Cr2O7)3 Recent user inquiry: 1/20/2024 18:59 Molar Mass Calculator Formula: C6H12O
SOLVED: The value of Ksp for PbCl2 is 1.6. What is the lowest concentration of Cl- that would be needed to begin precipitation of PbCl2 in 0.010 M Pb(NO3)2?
In the gas phase, PbCl 2 molecules have a bent structure with the Cl-Pb-Cl angle being 98° and each Pb–Cl bond distance being 2.44 Å. Such PbCl 2 is emitted from internal combustion engines that use ethylene chloride-tetraethyllead additives for antiknock purposes.. PbCl 2 is sparingly soluble in water, solubility product K sp = 1.7 × 10 −5 at 20 °C. . It is one of only 5 commonly The solubility product of PbCl2 298K is 1.7×10−5. Calculate the solubility of PbCl2 in g/lit 298K.Atomic weights : [Pb=207 and Cl=35.5]
![The solubility product of PbCl2 298K is 1.7×10−5. Calculate the solubility of PbCl2 in g/lit 298K.Atomic weights : [Pb=207 and Cl=35.5]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/SFkyRXpPWTdqMnc=/sd/)
Source Image: toppr.com
Download Image
How many formula units is 213 g of PbCl2? a 1.28 x 1026 b 4.61 x 1023 G 7.86 x 1023 d. 15.72 1024 – brainly.com In the gas phase, PbCl 2 molecules have a bent structure with the Cl-Pb-Cl angle being 98° and each Pb–Cl bond distance being 2.44 Å. Such PbCl 2 is emitted from internal combustion engines that use ethylene chloride-tetraethyllead additives for antiknock purposes.. PbCl 2 is sparingly soluble in water, solubility product K sp = 1.7 × 10 −5 at 20 °C. . It is one of only 5 commonly

Source Image: brainly.com
Download Image
⏩SOLVED:How many formula units are present in 500.0 g of lead(II)… | Numerade May 26, 2023= 207.2 + 70.9 = 278.1 g/mol Hence the Molar mass of PbCl2 is 278.1 g/mol. I hope you have understood the short and simple calculation for finding the molar mass of PbCl2. Remember In some books, you may see the unit of molar mass as grams/mole or g/mole. But all these units (i.e g/mol, grams/mole and g/mole) are the same.
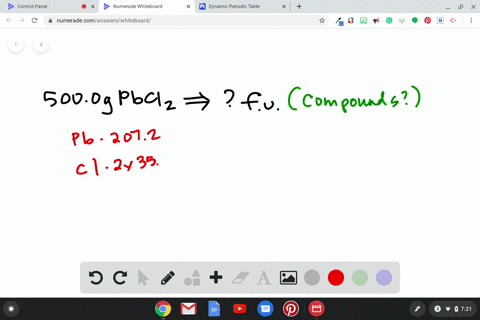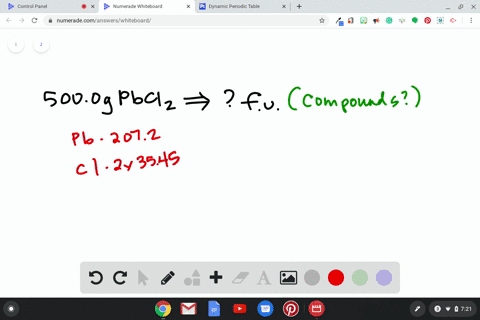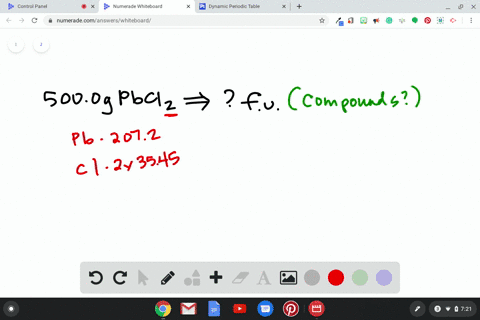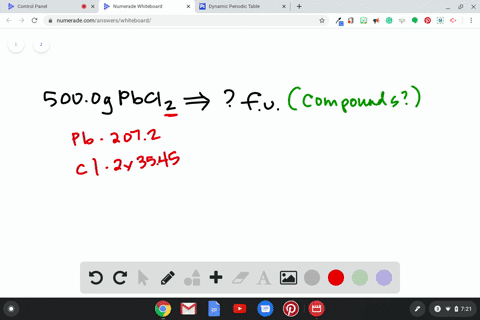
Source Image: numerade.com
Download Image
SOLVED: The Ksp for lead chloride PbCl2 is 1.6 x 10^-5. Calculate the solubility of lead chloride in each of the following: a) water Solubility: 0.0158 mol/L b) 0.27 M Pb(NO3)2 Solubility: In this video we’ll write the correct formula for Lead (II) chloride, PbCl2.To write the formula for Lead (II) chloride we’ll use the Periodic Table and foll
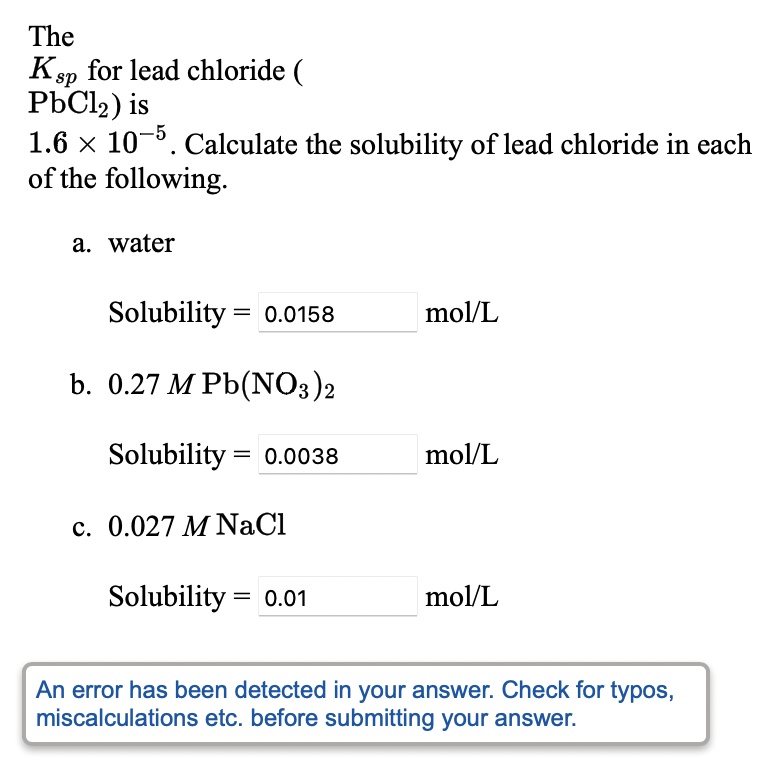
Source Image: numerade.com
Download Image
Calculate the molar solubility of PbCl2 in pure water at 25c. Ksp=1.17×10^-5 | Homework.Study.com PbCl2 = Pb + Cl is a Decomposition reaction where one mole of Lead Chloride [PbCl 2] decomposes into one mole of Lead [Pb] and two moles of Chlorine [Cl] Show Chemical Structure Image Reaction Type Decomposition Redox (Oxidation-Reduction) Reaction PbCl2 = Pb + Cl might be a redox reaction. Reactants Lead Chloride – PbCl 2

Source Image: homework.study.com
Download Image
Ksp of PbCl2 = 1.6 x 10 5 On mixing 300 ml, 0.134M Pb(NO2)2(aq) + 100 mL, 0.4 M NaCl(aq) 1. Q> Ksp 2.Q Structure, properties, spectra, suppliers and links for: Lead(II) chloride, 7758-95-4, PbCl2. Jump to main content Jump to site nav. Home; About us; Membership & professional community; Campaigning & outreach; Journals, books & databases … Molecular Formula Cl 2 Pb; Average mass 278.106 Da; Monoisotopic mass 277.914307 Da; ChemSpider ID 22867
 Download Image
Download ImageLead(II) chloride – Wikipedia Calculations: Formula: PbCl2 Molar Mass: 278.106 g/mol 1g=3.59575126031082E-03 mol Percent composition (by mass): Element Count Atom Mass % (by mass) Pb 1 207.2 74.5% Cl 2 35.453 25.5% Top Use: Molar Mass Calculator Formula: Co2 (Cr2O7)3 Recent user inquiry: 1/20/2024 18:59 Molar Mass Calculator Formula: C6H12O
Source Image: en.wikipedia.org
Download Image
How many formula units is 213 g of PbCl2? a 1.28 x 1026 b 4.61 x 1023 G 7.86 x 1023 d. 15.72 1024 – brainly.com
Lead(II) chloride – Wikipedia Mg2+Cl−Cl− (4.3.2) (4.3.2) Mg 2 + Cl − Cl −. Now the positive and negative charges are balanced. We could write the chemical formula for this ionic compound as MgClCl MgClCl, but the convention is to use a numerical subscript when there is more than one ion of a given type— MgCl2 MgCl 2.
SOLVED: The Ksp for lead chloride PbCl2 is 1.6 x 10^-5. Calculate the solubility of lead chloride in each of the following: a) water Solubility: 0.0158 mol/L b) 0.27 M Pb(NO3)2 Solubility: Ksp of PbCl2 = 1.6 x 10 5 On mixing 300 ml, 0.134M Pb(NO2)2(aq) + 100 mL, 0.4 M NaCl(aq) 1. Q> Ksp 2.Q PbCl2 = Pb + Cl is a Decomposition reaction where one mole of Lead Chloride [PbCl 2] decomposes into one mole of Lead [Pb] and two moles of Chlorine [Cl] Show Chemical Structure Image Reaction Type Decomposition Redox (Oxidation-Reduction) Reaction PbCl2 = Pb + Cl might be a redox reaction. Reactants Lead Chloride – PbCl 2